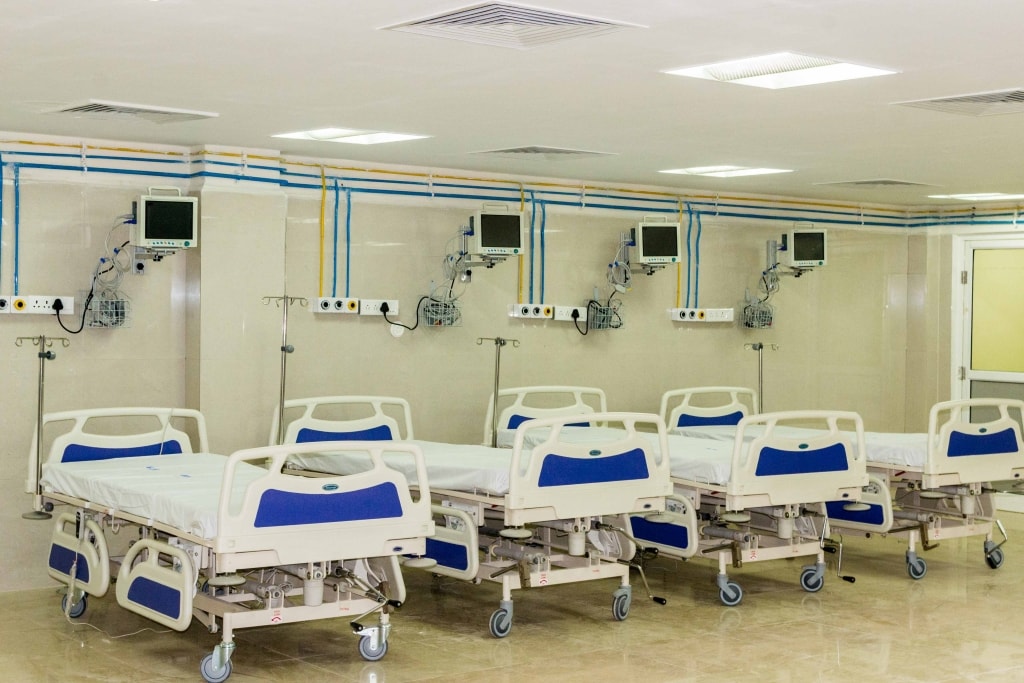
Modular Operation Theatre

There are basic policy decisions required for the construction of an operation theatre department and it is imperative that a high standard of discipline be maintained to minimize the incidence of infection and cross-infection. In order to determine this, the hospital department has been divided into zones as given bellow:
1. Protective Zone: The protective zone is the entrance area for patients, staff and supplies where normal hospital standards of cleanliness apply and where normal everyday clothes can be worn.
2. Clean Zone: In order to pass between the protective zone and the clean zone everything must undergo a system of transfer. This is the main area of the department and all patients, staff and supplies must be clean. A strict cleaning routine applies and everybody must undergo a complete changing routine to enter.
3. Aseptic Zone: The aseptic zone is the inner area where conditions are as near sterile as possible. It applies to two rooms in each suite: the theatre and the theatre supply room. All staff who might handle exposed instruments must be scrubbed and gowned.
4. Disposable Zone: In the disposable zone all exposed instruments (used or unused), pathological specimens, lotions, suction jars and soiled linen are passed from the theatre to a disposal corridor and returned for cleaning, sterilizing or any other necessary process.
Any hospital which has been a large operating department also has a great deal of traffic, to preserve the departmental character, a control system must be imposed over all people entering or leaving the hospital.
TECHNICAL SPECS OF A MODULAR OPERATION THEATRE
- a) In accordance with the customer preferences it can be provided in different antibacterial colors.
- b) The juncture of wall and ceiling and any screw heads are filled with special filler and a radius is provided to all the comers to ensure it is easy to clean and keep hygienic.
- c) The walls are constructed using special composite wallboard panels.
- d) The floor of the Operating Theatre is finished with Static Conductive PVC.
- e) The Doors are Semi Hermetic Sealing Sliding Doors.
- f) Air flow inside the Operation Theatre is provided by a Downward Laminar Flow System.
- g) A Stainless Steel wail mounted Storage Cabinet is provided within the Operation Theatre.
1. Air Handling Systems (with Laminar Air Flow)

Air handling system, OT interior & OT equipments. There are several options available in each part from where options are selected as per need, choice & budget. OT being most critical area in a hospital complex, it is necessary to control OT air in respect of temperature, humidity & particulate matter content of air, micro-organisms, i.e. bacteria, viruses, fungi etc.
Air handling systems process air through various stages. Air is cooled, moisture is removed, air is filtered to remove dust particles, bacteria, viruses; some air handling systems additionally remove gases, odors and other volatile organic compounds.
Laminar Air Flow is non-turbulent air flowing in parallel lanes vertically. LAF is filtered air coming over operative area from diffuser plenum above it. Plenum is fitted with HEPA (High Efficiency Particulate Air) filters of 0.3micron at 99.99% efficiency. Many times it is seen that plenum also works as light source above operative area; it has CFL/LED lights inside which provide clear shadow less light over operative area.
It is necessary to maintain particular air velocity in LAF so that filtered air coming over operative area carries bacterial load away from it. According to international standards it should be between 90-120 FPM (feet per minute). NABH (National Accreditation Board for Hospitals & Healthcare) also recommends the same.
Highly clean air of LAF falls in CLASS 100/ISO 5 category. When this air moves further in OT after leaving LAF area it mixes with less clean air of the room along with heat, moisture, bacterial load carried from operative area. It gradually looses it's clean state and while it reaches the last point in OT from where it is sent back to Air Handling Unit. Air in peripheral area of LAF normally falls in CLASS 10,000 - 100,000 categories depending on Air Handling Unit & OT size.
LAF can be made in various sizes. Apart from these standard sizes any other customized size can also be made.
Air Handling Unit has 3 filtration stages in total. First is 10 micron pre-filter, second 5micron fine-filter and third 0.3 micron HEPA filter. Out of three, first two are fitted in Air Handling Unit and last i.e. HEPA is fitted in plenum above operative area.
Fresh air is added to this within AHU. Positive pressure is created to restrict outside air entering OT though door etc. Necessary cascade pressure relief dampers are provided in wall to relieve excess pressure in hermetically sealed areas.
Air conditioning is done within Air Handling Unit to provide required cooling; no extra a/c is required in OT apart from this. HEPA plenum can be made in GI powder coat, Aluminum powder coat, SS304.
Specification of AHUs :
- 1.1. Type of Casing : Double Skin Polyurethane Foamed, Floor Mounted
- 1.2. Casing Details : Inner casing - 1.0 mm Aluminum / SS316 , Outer Skin : GI - 1.0 mm Power Coated
- 1.3. PUF Insulation Thickness : For outdoor Application 45mm Thick , 40 Kg/ m3 Density & for Indoor Application 25 mmThick,38 Kg/m3 Density.
- 1.4. Cooling Coil : 6 rows deep Copper Tube, Fins - Al. 12-13 FPI, Copper Tube Thickness shall be Minimum 0.5 mm.
- 1.5. Coil Casing-(Top/Side/Bottom ) : SS316
- 1.6. Drain Pan Material : SS 316
- 1.7. Drain Pan Insulation : 19mm Thick, Closed cell Nitrile Rubber,50 Kg/m3 Density.
- 1.8. Filter Frame where the Filter will be mounted : shall be Aluminum Heavy Gauge.
- 1.9. Pre-Filter & Microvee Filter Frame Material : Aluminum.
- 1.10. Fan : DIDW backward curved Centrifugal fan. 90 mm of water static pressure. Make : CG / Nikotra / Kruger.
- 1.11. Motor : Suitable for 415 + 10% volts, 50 cycle, 3 phase AC supply.
- 1.12. Fan & Motor Mounting : Heavy gauge Aluminum.
- 1.13. AHU Mouth Damper & Flange : Aluminum.
- 1.14. Provision will be made so that AHU cooling unit can be bypassed when required. For outdoor Application Thermal Break profile is recommended.
2. Walling & Ceiling (OT’s and Post Op.) :

Wall & Ceiling panels are of 80mm thickness and have a composite structure of one side EGP sheet and the other side powder coated GI sheet with PUF filler in-between. The panels will be made from 0.8mm EGP sheet with a PUF insulation of density 40 in between. The PUF will have a thickness of 48.6mm and GI panel of 0.6mm on the upper side. The total ceiling will have a thickness of 50mm. the ceiling will also have a PU antibacterial paint.
2.1. Facing Material:
EGP sheet will be finished with putty and a antibacterial antifungal coat to give a seamless coat.
2.2. Core Material:
A core of CFC frees rigid Poly Urethane Foam with typical physical properties:
- 2.2.1. Density of 40kg/m3.
- 2.2.2. Coefficient of conductivity < 0.025W/mK
- 2.2.3. Tensile Strength > 0.1N/mm2.
- 2.2.4. Compressive Strength > 0.1N/mm2.
- 2.2.5. Tensile Modulus > 3N/mm2.
- 2.2.6. Compression Modulus > 3N/mm2.
- 2.2.7. Dimensional stability at 800C and at - 200C < 2%.
- 2.2.8. Water Absorption < 0.55kg/m3.
2.3. Panel Properties:
- 2.3.1. Standard panels with reaction to fire classified as Cs2d0
- 2.3.2. Panels with superior reaction to fire classified as Bs2d0
3. Flooring : Conductive / Dissipative Flooring
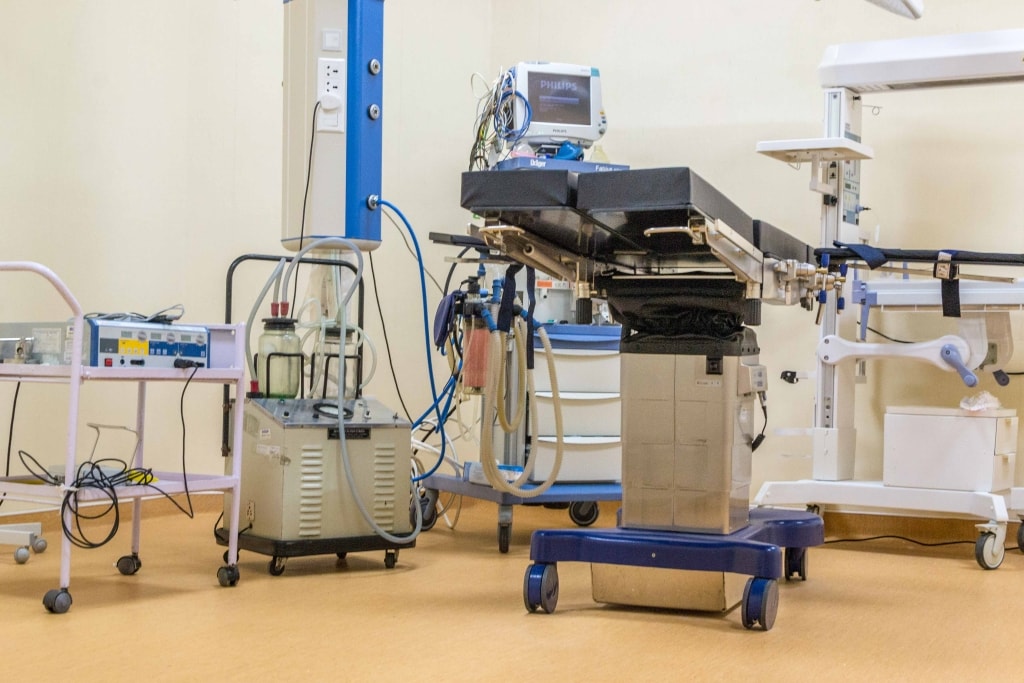
2mm Conductive flooring with Carbon backing having weight of 3kg/m². the product will be Poly Urethane reinforced (PU-Shield), scratch resistant, fire resistant, chemical resistant, slip resistant, antifungal, antibacterial growth, with a dimensional stability of &li; 0.4%, static electrical charges &li; 2KV, impact sound reduction (approx). The floor should have an electrical resistance of 2.5 x 10 to 10 6 Ohms.
4. Doors

4.1. Powder Coated Aluminum and Hermetically Sealing
To maintain sterility and the correct air pressure inside the rooms, all OT suite entrance doors would be of the sliding, hermetically sealing type. The door will meet following specifications:
- - Meets international quality and safety requirements.
- - Meets international quality and safety requirements.
- - Noise level of movement should not be more than 60 decibel
- - Controller should be microprocessor based and be CE marked and should have digital display.
- - The track should be made up of single piece extruded aluminum
- - Environment temperature 10ºC to 40º C.
- - Starting times should be able to regulate from 0.5 second to 23 second
- - Starting speed should be 600 mm per second.
- - Electrical safety codes for High & Low voltage system design should meet HTM 2020 /2021 standards.
The doorframe would be made of high quality anodized aluminum and the door panel will be made of compact laminate that can withstand high abrasion. To ensure efficient sealing of the doors, the door frames will be provided. They should consist of reinforced plasterboard panels faced with the same laminate as the doors. The door will seal on all four edges in the closed position & should be surface installed type. The track of the door will be constructed with an aluminum extrusion, fixed firmly to the walls. Nylon runner guides will be fixed to the floor in such a way that they do not obstruct trolley movement through the door. The doorframes will be edged with an aluminum extrusion and with concealed fixings that are adjustable during installation to ensure a 100% hermetic seal is achieved. Vision panels, 300mm x 300mm will be provided in the doors. The door controller will be sensing overload condition and in overload case the door will automatically stop & reverse the direction of travel. The controller will be capable of either being operated by elbow switches/foot switches, radar switch (touch less sensor). All doors will be able to be operated easily manually in the event of failure of the power supply or the automation unit.
CONTROLS AND SAFETY DEVICES.
The door shall be equipped as standard with two large size elbow-operated pushbuttons, a double pair of miniature 13 mm diameter safety sensors positioned to monitor the presence of obstacles on the threshold, back-up battery unit for emergency opening during power outages (the door stops in the open position in order to identify the presence of a fault in the electrical circuit).
4.2. Manual SS Single / Double Leaf Door (1500 x 2100 mm):
- 4.2.1. Type of Door : Outfitting / In fitting
- 4.2.2. Operation : Left/Right Hand (Pls indicate)
- 4.2.3. Door Frame Material : 1.2 mm (18 G) S.S. Sheet
- 4.2.4. Door Leaf Outer Material : 0.8 mm (22 G) S.S. Sheet
- 4.2.5. Door Leaf Inner Material : 0.8 mm (22 G) S.S. Sheet
- 4.2.6. Filler Material : PUF Insulation
- 4.2.7. Vision Panel (Size: 300 mm x 300), Double toughened glass
- 4.2.8. Door Seal : neoprene / EPDM.
- 4.2.9. Stainless Steel Hinge (100X75X3mm) (Make: ‘Dorset’) : 4 nos. hinges for Single door
5. Single Arm Moveable Pendant :
The Ceiling Pendant Systems designed to provide convenient positioning of medical equipment, medical gas terminal units, electrical and specialty services. The Ceiling Pendants will comply with NFPA 99, USA /HTM 2022. The support arms will be extremely robust and revolve on high quality bearings, so that the pendant head glides smoothly and quickly to any desired position.
The Pendant will have:
Single Arm (1000mm) with horizontal movement and load carrying capacity of 80 kg. The arm will be rotated up to 330 degree to 340 degree with adjustable stopper. The Pendant will have pneumatic brakes system will be adaptable to various safety requirements and construction facilities. Surgeon Pendant will have 1 arm with shelves as per points :
- 5.1. 5/15 Amp Electrical sockets without switches - 8 nos.,
- 5.2. Shelfs with side rails - 2 nos.,
- 5.3. Gas interface set for interface plate - 1 no.,
- 5.4. Ceiling mounting system for interim ceiling up to 1000
- 5.5. Interface plate with electrical fittings- 1no
- 5.6. Provision to fix 8 Gas Outlets
6. Operation Theatre Control Panel

The Surgeon Control Panel will meet Electrical Safety codes for High & Low voltage system, wired to the current IEE regulations. The room Surgeon’s Control Panel will be designed to cope with changing technology and equipment in operating environments. Control panel should be user friendly and ease of operating and maintaining purpose. The control panel will be of membrane type. The Control Panel shall be designed for front – access only. All internal wires shall be marked with plastic ferrule type cable markers, for ease of identification. The panel shall contain services as below :
- 6.1. Time Day Clock: Time Day Clock shall be digital type and clocks having high brightness characters.
- 6.2. Time Elapse Day Clock: Time Elapsed Day Clock shall be digital type and clocks having high brightness characters.
- 6.3. General Lighting System: General Lighting System shall incorporate all the necessary controls of the lighting system inside the theatre.
- 6.4. Medical Gas Alarm Panel: The medical gas alarm shall indicate High, Normal and Low gas pressure for each gas service present in the operating room and shall have an audible buzzer with mute facility. Pressure sensors shall be connected to MGPS for monitoring the pressures.
- 6.5. Hand Free Telephone set with memory card: A Hand Free Set Telephone system shall be incorporated in the panel with memory type card.
- 6.6. Temperature & Humidity Indicator: Temperature Indicator shall indicate the room temperature which should be connected to the local pressure switches of Air-Conditioning system. Indicator shall be digital type having high brightness characters, not less than 30mm in height.
- 6.7. OT Room Air Pressure Indicator
7. Medical Gases

Copper gas pipe line inside OT will be as per EN standard to cover Oxygen, Nitrous Oxide, Compressed air, Vacuum with accessories and CO2 Gas. The copper tube will be installed in conformity with the requirement of HTM.
Copper Piping – Technical Specifications and Testing Requirements
Copper pipes will be seamless solid drawn, half hard, tempered, phosphorus deoxidized, non-arsenical and degreased conforming to BS 2871-1971 (Part) Table X and chemical composition as per BS 6017 – 1981, Table 2, Cu-DHP of size. After laying the copper pipes the line will be tested for leaks at a pressure 1.5 times of working pressure. In case any pressure drop is observed in 24 hours, the same will be rectified and the test is repeated till there is no pressure drop for 24 hours. The pipeline will be supported at regular intervals with PVC saddles and painted with 2 coats of enamel paint as per International color codes.
8. Pressure Relief Dampers

Pressure Relief Dampers will be provided in each OT to prevent cross contamination of air from clean and dirty areas. Suitably sized air pressure relief damper will be strategically placed, enabling differential room pressure to be maintained and ensure that when doors are opened between clean and dirty area. Counter-weight balancing system will be provided in the PRD to maintain positive pressure inside the operation room. Air pressure stabilizers will have unique capability of controlling differential pressure to close tolerance. The PRD will remain closed at pressure below the set pressure and should open fully at pressure only fractionally above the threshold pressure. The body will be epoxy powder coated as per standard BS colors. First class electrolyzed steel plate will be used for body and with high grade SS304 Stainless Steel for blades.
9. Fusible link Type Fire Dampers (if applicable) :

- 9.1. All supply and return air ducts at AHU room crossings and at all floor crossings shall be provided with approved make fire dampers of at least 90 minutes fire rating.
- 9.2. Fire damper blades and outer frame shall be formed of 1.6 MM galvanised sheet steel. The damper blade shall be pivoted on both ends using chrome plated spindles in self lubricated bronze bushes. Stop seals will be provided on top and bottom of the damper housing made of 16 G galvanised sheet steel. For preventing smoke leakage side seals will be provided. In normal position damper blade shall be held in open position with the help of a spring held in position by fusible links of approved rating thereby providing maximum air passage without creating any noise of chatter.
- 9.3. The fire damper shall close due to temp rise in SA. Ducts thru the fusible links factory set at 165 Deg. F. micro switches with bakelite base will be provided to stop fan motor and give open and close signal at remote panel in case of fire.
- 9.4. The fire dampers shall be mounted in fire rated wall with s duct sleeve 600 MM long. The sleeve shall be factory fitted on fire damper. The joints at sleeve end shall be slip on type. Minimum thickness of GI sheets shall be 18G.
10. Supply/ Return Air Diffusers
- 10.1. Diffusers shall be of approved make and of aluminum construction, round in shape with flush fixed pattern or adjustable flow pattern. Diffusers for different spaces shall be selected in consultation with the Consultant. All supply air diffusers shall be equipped with removable key operated volume control dampers. Anti - smudge ring will be required in specific applications. All extruded aluminum diffusers shall be provided with removable central core and concealed key for operating the volume control damper. The main extruded section shall be minimum 15 mm wide.
- 10.2. Linear diffusers shall be extruded aluminum construction one or two way blow type. The supply air diffusers shall be provided with volume control dampers to be fitted within the supply air collar.
- 10.3. Slot diffusers shall be extruded aluminum construction multi slot type complete with Hit and Miss volume control damper.
11. Supply/ return air grills :

Grills shall be of extruded aluminum construction having concealed screws for fixing. These shall be duly powder coated in the shade approved by TMH/ TSL. The grills shall have horizontal bars at 15/30 deg. inclination and flanges on top and bottom. The grills at the end shall have flange on the side as well as per the site requirement. The size of main extruded section for flanges shall be minimum 15 x 15 mm and the louvers shall be 15 mm deep having thickness of mm in the front and 2.8 mm at the rear.
12. Aluminium Ducting with Nitrile Insulation
Ducting shall be made of Aluminum for supply & for return with curves and bends where indicated for easy flow of air and ensured to be air tight by applying Silicon sealant after fabrication .hanger shall be provided to ducts & shall be suspended by means of GI coated rods & these shall not be more than 2.5mtrs apart .Thermal Insulation with 12mm XPE with aluminum foil for supply & return air ducts. Joints should be lapped with Nitrile rubber tape for better insulation.
13. Plenum with Mini Pleat / Conventional HEPA Filters :
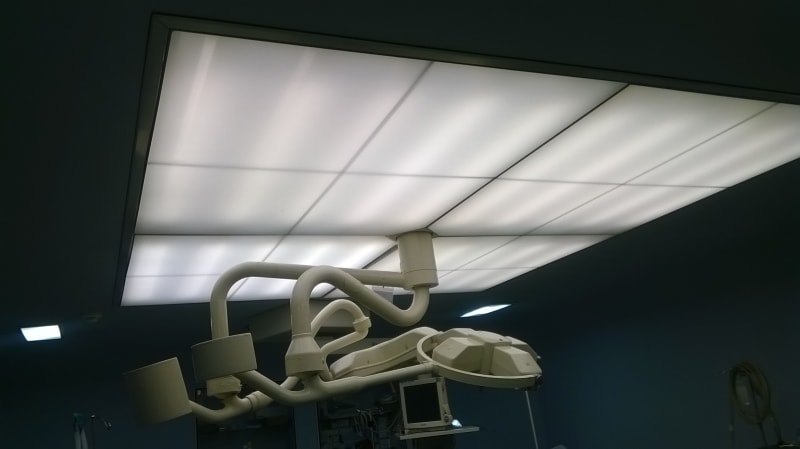
The Caracas of the plenum will be made of extruded aluminum sections in such a manner that the air is passed only through the filters only. Plenum unit for laminar flow diffuser will be made of thick aluminum sheet. The complete unit will have factory prepared fine sealing system. The plenum will be supplied at site duly sealed in factory made packing. The Laminar flow system will have anodized aluminum perforated diffuser grill. The laminar flow system will have such design that it provides cleanliness of class 1000.
All filters are having genuine fiber Glass media manufactured in Europe or USA (either by Lydall Corporation or Hollingsworth & Vose), not the cheapest one manufactured in China. So, the life and efficiency of the filters are nearly double in comparison to available so called filters in Indian market whose country of origin in China.